For OEMs establishing the future generation of minimally invasive medical gadgets, the interior cable assembly is a crucial frontier. While ultra-fine coax cables allow the high-density signal transmission important for advanced endoscopes, IVUS, as well as ICE systems, their efficiency in the medical atmosphere is determined through two non-negotiable requirements: biocompatibility and sterilization protection. A cable that provides ideal signal stability in the laboratory, however, stops working in the autoclave or even poses a client danger, is pointless. Medical-grade ultra-fine coax is crafted particularly to satisfy this double requirement, where product scientific research is as essential as electrical design.
The Foundation: Material Selection for Direct and Indirect Patient Contact
Biocompatibility is certainly not a single material property, but a methodical recognition of product security. Medical cables are categorized by their contact kind: some, such as parts of an endoscope cable or even medical scalpel cable, might have indirect exposure to client cells or even fluids, others, such as specific oral sensing cables or even leads, might have direct contact. Medical-grade assemblies solely utilize polymers as well as steels, along with shown biocompatibility, like particular qualities of fluoropolymers (FEP, PTFE), polyurethanes, and silicones for protection as well as jacketing. These products are developed without phthalates or heavy-metal stabilizers that might leach out. For OEMs, this implies choosing a cable companion along with deeper proficiency in accredited, deducible product source chains, guaranteeing every element satisfies ISO 10993 and USP Course VI requirements.

Withstanding the Cycle: Resistance to Repeated Sterilization
Medical gadgets are subjected to extensive sterilization techniques to avoid infection. Each technique provides a distinct product difficulty:
Autoclave (Steam Sterilization): High-pressure filled heavy vapor at 121°C to 134°C is one of the most typical yet aggressive sterilization methods. It can easily activate hydrolysis in regular plastics, resulting in jacket breaking, protection embrittlement, and ultimate electrical failure. Medical-grade cables utilize specific, hydrolysis-resistant polymers that preserve versatility as well as dielectric properties after numerous cycles.
Chemical Sterilization (EtO, Hydrogen Peroxide Plasma): These techniques assault products in a different way. Ethylene Oxide (EtO) can easily penetrate as well as deteriorate specific adhesives and polymers, while plasma can easily trigger surface oxidation. Cable building should utilize suitable products as well as guarantee no secured pockets catch chemicals, which might later on outgas as well as hurt clients or even devices.
A cable for a recyclable ultrasound probe or even robotics cable harness should be developed from the beginning for a defined lifetime of sterilization cycles, a demand missing from consumer AR/VR cables or even drone harnesses.

Maintaining Performance Under Environmental Stress
Real examination of a medical-grade cable is its own ability to preserve electric stability with the integrated stresses of sterilization as well as medical use. Duplicated thermal cycling can easily delaminate guards, change dielectric constants, and compromise solder joints at connectors. This can easily lead to:
Impedance Drift: Triggering signal representations as well as reduction of picture clarity in 4K endoscope systems.
Increased Signal Attenuation: Decreasing the level of sensitivity of indicators coming from EEG top cables or even ICE catheters.
Shield Degradation: Lifting the danger of electromagnetic interference (EMI) in delicate atmospheres.
For that reason, screening should exceed initial electric specs to consist of post-sterilization efficiency recognition. The ultra-fine coax in a medical gadget should be a steady, foreseeable element throughout its whole life span.
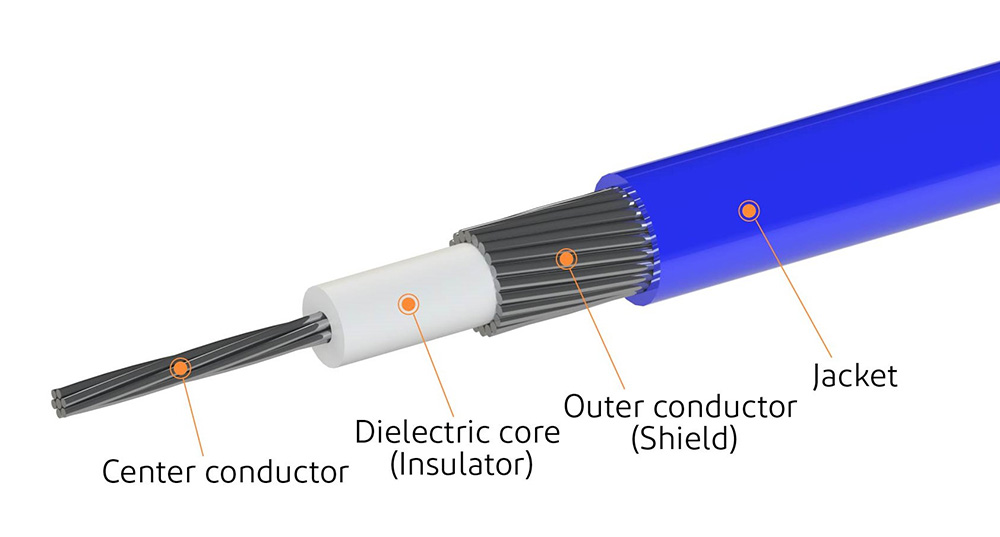
System-Level Design for Safety and Reliability
Biocompatibility as well as sterilization durability prolong past the cable to the whole setup. This includes:
Sealed Connectors and Overmolds: Joints should be hermetically secured utilizing medical-grade adhesives as well as overmolds to avoid liquid ingress, a crucial failing factor for RF ablation cables or even endoscope links.
Strain Relief Design: Bend factors should be crafted to withstand the stiffening or even breaking that can easily happen after repeated warm and chemical exposure, safeguarding the great conductors within.
Full Traceability and Documentation:OEMs need to finish paperwork (Material Declarations, Certificates of Conformance, test reports) for regulatory submissions (FDA, MDR). This traceability guarantees responsibility for each product utilized in the setup.

For OEMs, the cable is an important subsystem that brings all information, as well as danger. At Hotten Electronic Wire Technology, our team designer our medical-grade ultra-fine coaxial assemblies using this extensive obligation in thoughts. Coming from the choice of accredited biocompatible products to the recognition of every design versus sterilization procedures, our team develops cables that satisfy the strict needs of medical, oral, and surgical applications. Our proficiency guarantees your ingenious gadget certainly not just advancement efficiency but also the important benchmarks of security, reliability, as well as regulative conformity.
 Hot News
Hot News2025-12-17
2025-12-11
2025-12-05
2025-04-29